
OTTAWA – A commonly used product used on Metrolinx and Go Transit to help curb COVID-19 has Health Canada contacting everyone who has stated online that they are using the product AegisMicrobe Shield.
Health Canada has done a Google search on companies and organizations that has been using AegisMicrobe Shield.
In an email provided to NetNewsLedger written by Health Canada officials it states:
“Health Canada administers the Pest Control Products Act (PCPA) and its Regulations. The main objective of the PCPA and its regulations is to protect human health and the environment and prevent unacceptable risks by regulating products used for the control of pests. All pesticides manufactured, imported, distributed, or used in Canada are regulated to ensure they meet Canadian health and environment standards, bear the Canadian label with a Pest Control Product Registration Number, and are used according to label directions.
“Health Canada regulates disinfectants and sanitizers under two separate Acts and frameworks, the Food and Drugs Act (FDA) covers disinfectants and the PCPA covers sanitizers. AEM 5700 Antimicrobial, PCP Reg. No. 15133, otherwise known as AegisMicrobe Shield (US EPA registration), is approved for use as a material preservative [i.e., to impart durable, broad-spectrum, antimicrobial protection to existing non-food contact building surfaces (porous or non-porous) to control and/or prevent microbial growth].”
The product has not been approved for use against COVID-19.
This product is what Metrolinx and Go Transit and other transportation companies were using since March 2020.
The application of the long-acting anti-microbial continues on our GO fleet. Crews completed UP Express trains last night. Doing whatever we can to protect you & your loved ones from #COVID-19 pic.twitter.com/LM4e1ipnxP
— Anne Marie Aikins (@AMAwithAMA) March 4, 2020
“Health Canada has not received an application for registration of AEM 5700 or Aegis Microbe Shield as a disinfectant or as a sanitizer, and therefore its use as such has not been reviewed or approved by Health Canada. Please note that when these products are advertised or used for the purpose of mitigating or preventing disease in humans or animals, then these products also fall subject to the FDA.
“Health Canada is requesting that any false or misleading claims, particularly any claims respecting how the product can be used against SARS-COVID 19, be removed from websites and any social media platforms immediately.”
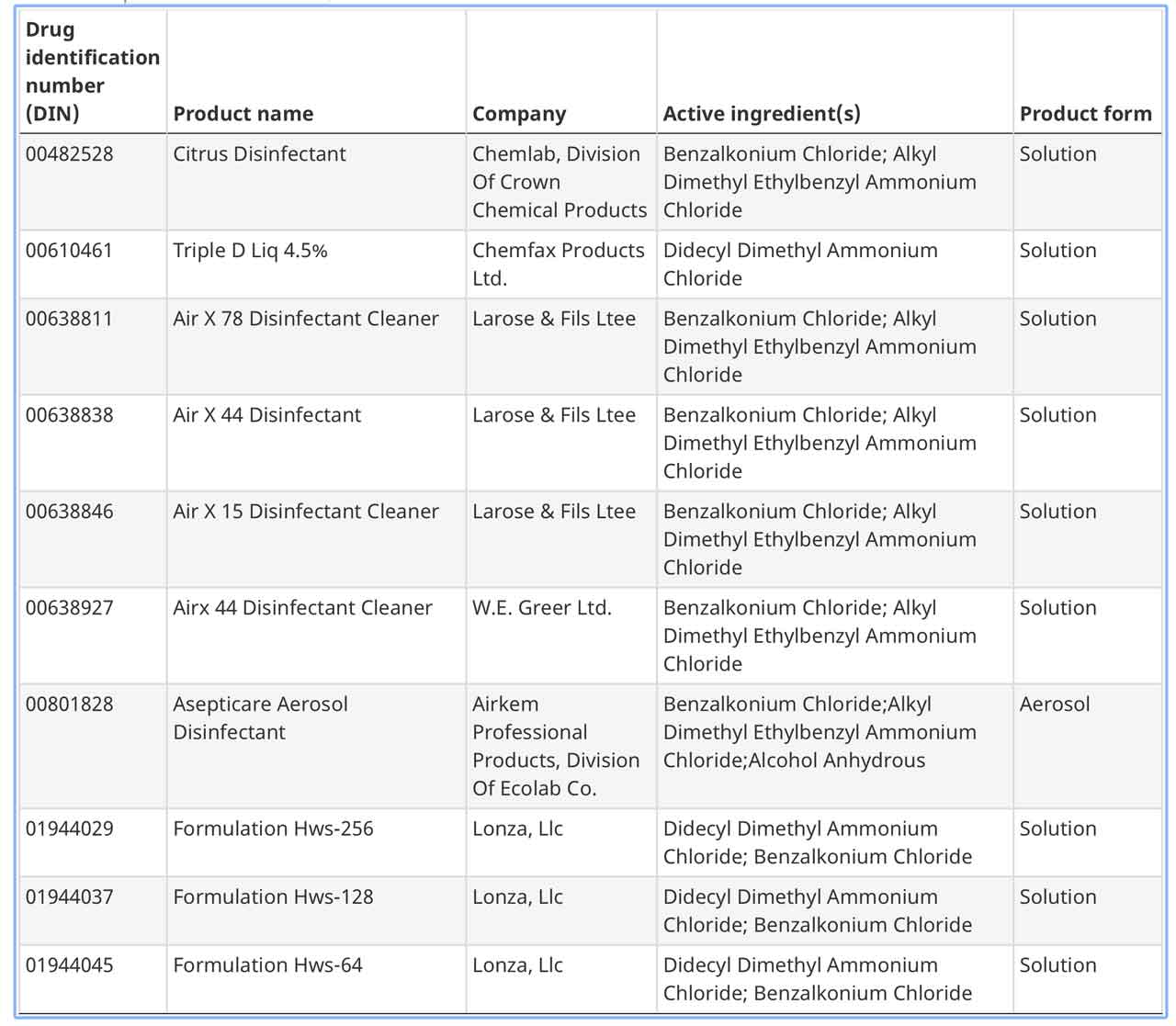
Here is a list of the products approved for COVID-19 by Health Canada.
Here is the first page of approved products, Click the link above for the full list.
Developing…






