
PHILADELPHIA – Stem cell research is impacting medical science, and the lives of millions of people. Now stem cell research appears to be helping to explain the origin of life on land on Earth. “We asked if these progenitor cells are capable of generating both heart and lung derivatives,” says Edward E. Morrisey, PhD, professor of Medicine and Cell and Developmental Biology and scientific director of the Penn Institute for Regenerative Medicine. “Our data show that Wnt2-positive cells exist prior to lung development and help coordinate lung and heart co-development by generating cell types in both tissues.”
“It’s pretty obvious to anyone who has looked at the anatomy of most terrestrial animals that the heart and lung are intimately linked. This is even reflected in clinical medicine where in many places, including the Perelman School of Medicine, the Division of Cardiovascular Medicine was once referred to as the Division of Cardiopulmonary Medicine,” notes Morrisey.
Biologists have known that the co-development of the cardiovascular and pulmonary systems is a recent evolutionary adaption to life outside of water, coupling the function of the heart with the gas exchange function of the lung. And, the lung is one of the most recent organs to have evolved in mammals and is arguably the most vital for terrestrial life.
Evolution of Life Explained by Stem Cell Research
The evolution of adaptations for life on land have long puzzled biologists – are feathers descendents of dinosaur scales, how did arms and legs evolve from fins, and from what ancient fish organ did the lung evolve?
The coordinated maturation of the cells of these two systems is illustrated during embryonic development, when the primitive lung progenitor cells protrude into the primitive cardiac progenitor cells as the two organs develop in parallel to form the cardiopulmonary circulation. However, little is known about the molecular cues guiding this simultaneous development, and how a common progenitor cell for both organs may influence the pathology of such related diseases as pulmonary hypertension.
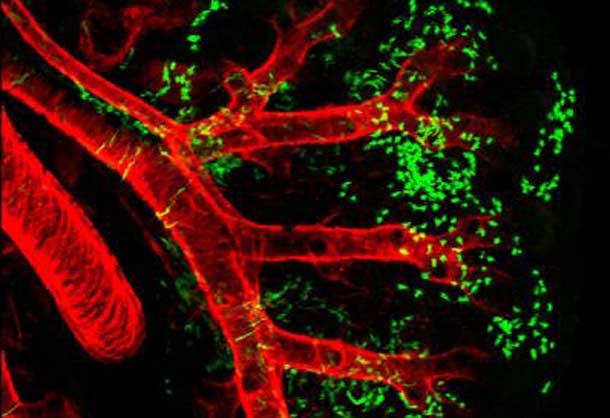
In a new paper published this week online in Nature, a team from the Perelman School of Medicine, at the University of Pennsylvania, illustrates how pulmonary vasculature, the blood vessels that connect the heart to the lung, develop even in the absence of the lungs. Mice in which lung development is inhibited still have pulmonary blood vessels, which revealed to the researchers that cardiac progenitors, or stem cells, are essential for cardiopulmonary co-development.
The Penn team, led by Edward E. Morrisey, PhD, professor of Medicine and Cell and Developmental Biology and scientific director of the Penn Institute for Regenerative Medicine, identified a population of multi-potent CardioPulmonary mesoderm Progenitor cells they named CPPs. The CPPs can be distinguished from many other early embryonic cells by the expression of a well-studied signaling molecule Wnt2. The issue of how the lung develops and connects to the cardiovascular system has intrigued the Morrisey lab for many years.
The Morrisey lab began with a couple of simple questions: how do the lung and heart co-develop and what are the critical signals that regulate this process? The breakthrough in this work occurred when the team characterized the expression pattern of the Wnt2 gene.
WNt2 Gene Unique in Early Life
“Wnt2 is expressed in a unique place in the early embryo — exactly in between the early heart and foregut tube, where the lung will arise from.” This allowed the researchers to create a model system in mice, whose cardiopulmonary anatomy is very similar to humans, and ask whether Wnt2-positive cells could coordinate heart and lung co-development.
Using cell lineage tracing analysis, they showed that Wnt2 cells generate single clones that, in turn, generate both heart and lung tissue, including cardiomyocytes and blood vessel cells such as vascular smooth muscle. Indeed, CPPs are capable of generating the vast majority of early embryonic cell types in the heart and lung. These studies also showed that the different cell lineages within the lung are related. For example, vascular smooth muscle and airway smooth muscle share a common progenitor cell in the lung.
The development of CPPs is regulated by the expression of another well-known protein called hedgehog, which is required for proper connection of the pulmonary vasculature to the heart. These studies show that hedgehog, which is also expressed by early lung progenitor cells, helps to promote CPPs to differentiate into the smooth muscle component of the pulmonary vasculature.
Together, these studies identify a novel population of multi-potent cardiopulmonary progenitors that coordinate heart and lung co-development, which is required for adaptation to terrestrial existence.
The finding that CPPs coordinate lung and heart co-development also has important implications for diseases that affect both organs, such as pulmonary hypertension. It is unclear whether pulmonary hypertension is primarily a lung disease or whether there are also intrinsic defects in the heart or cardiovascular system. The identification of CPPs could provide important insight into pulmonary hypertension and other diseases by identifying a common progenitor cell for both organs. Future studies will focus on whether CPPs exist in the adult cardiopulmonary system and whether they play a role in the response of the lung and heart to injury or disease.









