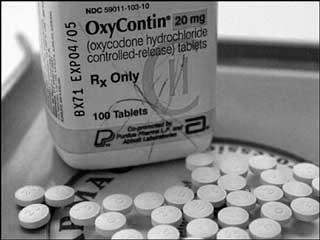
 THUNDER BAY – The decision by federal Minister of Health Aglukkaq to allow generic OxyContin into Canada has brought down critical commentary from both Ontario and NishnawbeAski Nation. Deb Matthews, Ontario Minister of Health and Long-Term Care stated, “I am profoundly disappointed in Minister Aglukkaq’s decision to ignore the threat to public safety posed by generic OxyContin and to allow it to enter the Canadian market. National problems require a national solution. Provincial and territorial health ministers unanimously asked for federal help, but have once again been told that it’s a provincial problem.
THUNDER BAY – The decision by federal Minister of Health Aglukkaq to allow generic OxyContin into Canada has brought down critical commentary from both Ontario and NishnawbeAski Nation. Deb Matthews, Ontario Minister of Health and Long-Term Care stated, “I am profoundly disappointed in Minister Aglukkaq’s decision to ignore the threat to public safety posed by generic OxyContin and to allow it to enter the Canadian market. National problems require a national solution. Provincial and territorial health ministers unanimously asked for federal help, but have once again been told that it’s a provincial problem.
“The most effective way to prevent a renewed addictions crisis is to ban generic OxyContin entirely. That’s why in July, I wrote Minister Aglukkaq to urge her to use her powers to protect public safety and ban generic OxyContin from entry into Canada. The prospect of making a cheaper formulation more widely available is a matter of grave concern, threatening the safety of individuals and the population at large. If the Government of Canada had truly wanted to do what was in the best interests of all Canadians, it could have stopped these drugs from being readily available”.
“Prescription OxyContin has wreaked havoc on too many Ontario families. It is associated with a five-fold increase in oxycodone-related deaths and a 41 per cent increase in overall opioid-related deaths. The social costs of allowing generic OxyContin have been estimated at $500 million a year. Patients who legitimately need pain medication have many other options, including OxyNeo, which addictions experts have found to be tamper-resistant,” continued the Minister. “Police chiefs and pharmacists agree that generic OxyContin poses a public safety threat and should be banned. I urge the Federal Minister of Health to reconsider her decision, and do what is best for the health of Canadians. I’m asking other concerned Canadians to join me in this call”.
Nishnawbe Aski Nation (NAN) Deputy Grand Chief Alvin Fiddler is calling today’s announcement by Health Canada to open the market to drug manufacturers to create generic, less expensive OxyContin, another blow to the northern remote First Nations who are currently combating an addiction epidemic. “With OxyContin clones on the market, it just means more drug flow to the north. NAN is disappointed with Federal Minister Aglukkaq’s refusal to intervene in the approval of generic versions of the drug,” said Deputy Grand Chief Fiddler. “While we appreciate the Minister’s distinction between science and politics, NAN First Nations are experiencing extreme levels of addiction and require extreme solutions.”
Deputy Grand Chief Fiddler urges the Minster to delay the decision until more research is done, with an emphasis on the potential social impacts of the generic drug’s approval.
First Nation groups, police organizations, addiction experts, health-advocacy organizations and the governments of Ontario strongly oppose generic versions of OxyContin in Canada because they believe the drug is fuelling a nationwide problem.
“Our Government is taking action to tackle prescription drug abuse,” said Minister Aglukkaq. “This is a serious issue that destroys the lives of individuals and families, and I believe we have the responsibility to work with the provinces and territories to address it head-on.”
Under the authority of the Controlled Drugs and Substances Act (CDSA), Health Canada will now impose tough new conditions on the licences of dealers who manufacture and distribute products that contain the controlled release formulation of oxycodone. For example, dealers will be required to report spikes in sales and changes in distribution patterns, in addition to Health Canada’s current requirements to report loss and theft. If evidence of abuse is uncovered, action can be taken, up to and including the revocation of their licence to deal in certain types of medications. If required, matters could be referred to legal authorities.
“Real action is required at the provincial and territorial level, too,” added Minister Aglukkaq in the letter.
Federal action is only one component of tackling prescription drug abuse. Medical practitioners who prescribe drugs fall under provincial and territorial jurisdiction. Accordingly, Minister Aglukkaq strongly encouraged provinces and territories to speak with their local medical associations about the topic, and to strengthen provincial and territorial practices to fight prescription drug abuse, which include establishing training requirements, setting scopes of practice for physicians and other practitioners, and monitoring prescription practices. Similar controls, including a prescription monitoring program, have been applied by Health Canada in recent years to the Non-Insured Health Benefits program for First Nations and Inuit, which is administered by the federal government.
There is no basis in the Food and Drugs Act for the Minister of Health to withhold approval of a drug where the drug is otherwise considered safe and effective for its recommended use. The law does not permit approval to be withheld on the basis of misuse. In the letter, Minister Aglukkaq confirmed that she would not politically interfere with Health Canada’s scientific review process concerning generic controlled release formulations of oxycodone.



